By Asa Waldstein
Practitioners selling dietary supplements can easily cross the line into FDA/FTC trouble. This article reviews common marketing mistakes and discusses lower-risk ways to sell supplements while maintaining a thriving practice.
We often hear about not making disease claims, but it can be confusing to know what a claim is. Here are a few general rules.
WHAT IS A CLAIM?
- Anything ending in “itis” refers to inflammation-of, such as colitis
- Anything a drug is “indicated for”, such as drugs being indicated for diarrhea
- Most things with an “anti” in the name, such as anti-viral
- The name of any disease, such as Crohn’s Disease
- Am I making a statement that can be construed as treating, diagnosing, preventing, or curing any disease?
Education Versus Marketing
Education is okay, but there is a thin line between educating about health concerns and making uncompliant marketing claims. Here are a few scenarios.
Scenario #1
Citing ingredient research and benefits.
● Low Risk: A doctor’s website discusses the dangers of high blood pressure and provides information about alternative therapies such as diet, exercise, and meditation. There is also research on ingredients used for high blood pressure.
● High Risk: This scenario above becomes a high risk if the doctor’s website sells supplements for high blood pressure or contains discussed ingredients. For example, discussing ingredient research is a marketing claim if that ingredient is contained in any product sold on the website.
Scenario
#2 Testimonials
● Low Risk: Client testimonials do not refer to supplements and only reference the therapeutic nature of the practitioner’s practice, such as dietary interventions and acupuncture results.
● High Risk: This same scenario above becomes a high risk if the client testimonials include product reviews with disease words such as “Product ABC worked great for my arthritis.”
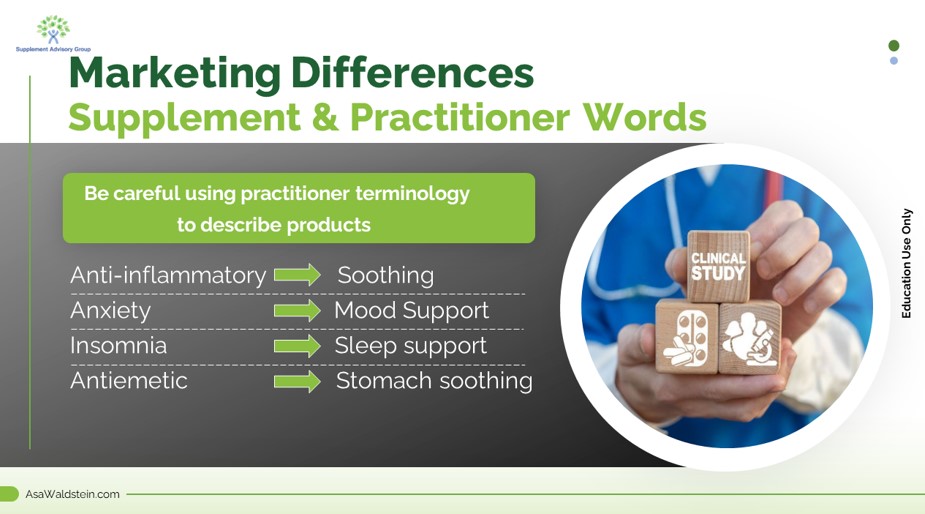
Disease Words
Practitioners are accustomed to using medical and drug terminologies such as anti-inflammatory, hypertension, or anti-viral in their practice. However, these words do not translate into compliant marketing, and it’s a common practitioner mistake to use these risky words to describe supplements. If truthful and not misleading, Here are some alternative ways to discuss these words.
Follow Enforcement Trends
Following the ever-changing enforcement trends is a great way to ensure dietary supplement marketing is compliant. One example is the word “hangover,” which would have been a relatively low risk in the past, but in July 2020, the FDA issued seven warning letters to companies making hangover claims. Companies that were paying attention removed this word from their online marketing and social media, thus significantly reducing their risk of a future warning letter. The FDA is looking at several-year-old blogs and social media posts in the same manner as current posts.
I recently conducted a review of diabetes and blood sugar-related FDA warning letters. Since January 2021, there have been 56 diabetes-related letters. These findings provide a snapshot of where the FDA is finding and perhaps looking for risky words. We can learn a lot from this, and it’s an excellent reminder for companies to re-evaluate these areas to ensure no disease statements are lurking on their website or social platforms. Here is what I found.
●62% include claims made on social media. This is an excellent reminder to scan for high-risk words on socials, including old posts.
●26% involve claims made in blogs. This is a strong enforcement trend to watch as the FDA continues to cite claims made in blogs. Many of these blogs may be “forgotten”on company websites. All marketing material on a commercial website is “fair game.” This is an important reminder to scan all blogs for risky words such as diabetes, cancer, and depression.
●23% include claims made in testimonials.
●25% involve claims made in hashtags.
A good resource to stay on top of trends is my weekly Warning Letter Wednesday post (https://www.asawaldstein.com/warning-letter-wednesday), where I review interesting FDA enforcement trends.
What Are the Repercussions of Not Complying
The most common agency action is a warning letter from the FDA, FTC, or sometimes both. Here are some reasons to avoid a warning letter.
- Requires legal resources to respond
- Public record
- Repeat warning letters can lead to an injunction
- Scares away investors and partnerships
- Alerts class action attorneys
Too Small to Be on the FDA’s Radar
Some companies feel they are “too small” to be on the FDA’s radar. Based on numerous practitioner warning letters, this is untrue. One example of this is a recent warning letter that mentioned a claim made in a YouTube video. When I reviewed this video, it only had about 60 views, demonstrating that the authorities may scrutinize any marketing.
Best Practices for Compliant Marketing
Step one in reducing risk is to remove risky words from all marketing platforms. If accurate, finding truthful ways to talk about products without disease words is a good next step. For example, replacing “anxiety” with “balanced mood support” and “anti-inflammatory” with “discomfort” may be suitable options. The key to compliant marketing is to make the point without disease words while being truthful. This can be done by using substantiated structure-function statements such as “supports heart health” or talking about a quality-of-life discussion about “feeling resilient” or “refreshed in the morning.”

Asa Waldstein is a 20-year dietary supplement executive now focusing on bridging the compliance, marketing, and regulatory gap between the supplement and hemp industries. Asa also is the principal of the consulting company Supplement Advisory Group, a boutique group focusing on marketing risk analysis and practical marketing solutions for the web and social media. Waldstein is chair of the American Herbal Products Association’s (AHPA) Cannabis Committee, and his Regulatory Education Series platform regularly hosts free events for the community. Learn more and contact at AsaWaldstein.com.











